TECHNICAL DATA SUMMARY
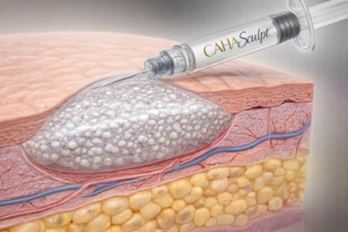
- Composition: 30% Calcium Hydroxyapatite (CaHA) microspheres; 70% Aqueous Gel Carrier.
- Microsphere Precision: Smooth, spherical particles (25–45 μm) designed to optimize biocompatibility and prevent distal migration [1].
- Rheology: High G-Prime (viscoelasticity) provides maximum projection and resistance to dynamic facial forces.
- Non-Hydrophilic: Formulated to not attract water, ensuring immediate, predictable correction without post-procedural edema [2].
CLINICAL APPLICATIONS: FACE & HANDS
- Facial Architecture: Targeted for the deep dermal and subdermal correction of volume loss and the definition of the jawline, chin, and mid-face.
- Hand Restoration: Provides a smooth, uniform volume replacement on the dorsum of the hands, effectively masking tendons and veins [3].
- Biostimulatory Versatility: In its hyper-diluted form (1:1+), the product functions as a skin-quality architect, stimulating the production of Type I Collagen and Elastin to improve skin laxity [4].
STRATEGIC PARTNERSHIP & WHITE LABELING
- Advasaf provides a streamlined path for global partners to launch premium USA-made injectables.
- Quality Systems: Manufactured in FDA-registered facilities under ISO 13485:2016 standards.
- Custom Branding: Available for white-labeling and localized branding to fit regional market identities.
REGULATORY NOTICE: CAHA SCULPT™ is currently in the developmental phase and is not yet commercially available. Technical claims are based on established scientific benchmarks for Calcium Hydroxyapatite technology.