How do I do it? Starting to think about sterilization.
Navigating how to produce a sterile product can be seen as a challenging process but let's break it down into steps for your consideration. Some of these items may be done in parallel (e.g. selection of sterilization provider along with packaging definitions). While the sterilization aspires to be perfect science, the process to develop the process for your product can be a trial and error if one doesn't have experience.
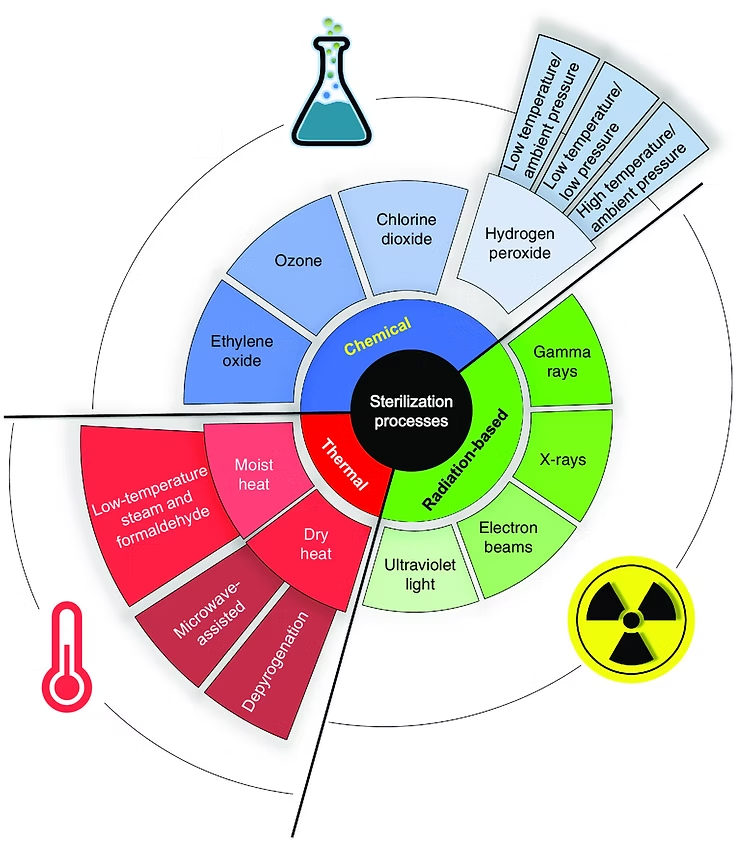
Here is a visual from Sterilization of Objects, Products, and Packaging Surfaces and Their Characterization in Different Fields of Industry: The Status in 2020
Here are the steps we propose:
1. Modality/Method Choice... What method can I use? - if you look at the product materials your device is made out of this often indicates the best method to use.
Many materials drive the sterilization method. For instance Steam and dry heat require materials that can withstand high temperatures. Some products degrade by certain processes such as UV light and Irradiation. Sometimes you can adjust parameters to make a certain material work such as temperature or actual amount of exposure. For traditional methods there is a lot of published data and ISO standards around that can help.
2. Packaging and its compatibility with sterilization method.
3. Labeling to ensure conformance during sterilization.
4. Contract Sterilizer selection, qualification, registration (FDA), commercial agreements.
5. Validation of the sterilization process to document effectivity and lethality.
6. Shelf Life claims determination (applicable to medical devices).
7.Regulatory submissions and approvals.

Reliability
Share your experiences or queries below. Your feedback is highly valued and will help improve our content.

Assistance
If you're feeling overwhelmed or uncertain, reach out! We're here to assist you every step of the way.

Scalability
Get in touch

WhitneyCaine
Aug 27, 2023
Comments
I like the break down of the sterilization process, it is something that is difficult to understand.

Scalability
Like

ToriDaniel
This is a great article and is something more sterilization companies should address.
Aug 26, 2023
Consistent performance and uptime ensure efficient, reliable service with minimal interruptions and quick response times.

Scalability
Consistent performance and uptime ensure efficient, reliable service with minimal interruptions and quick response times.

jalenmscrowensen980
Aug 24, 2023
Best sterilization process?
Apply to non-medical devices?

Reply
Like

oiasjdfo
Aug 23, 2023
What is the best sterilization method for orthopedic devices?
Like

Scalability
Reply

Is EO undergoing changes?
Consistent performance and uptime ensure efficient, reliable service with minimal interruptions and quick response times.
oiasjdfo
Aug 23, 2023

Like
Reply

aoisjdfoia
Aug 23, 2023
Dos shelf life vary depending on the sterilization method?
Like

Scalability
Reply

aoisjdfoia
What are your thoughts on the recent developments of EO?
Aug 23, 2023
Like

Scalability
Reply

oaijfoiasj
Aug 23, 2023
Like
EO seems like it may not be around or will have to undergo massive changes.

Scalability
Reply

oaijfoiasj
Sterilization is a lot more complex than I imagined. Makes sense why companies take so long to return products sent for sterilization.
Aug 23, 2023
Sterilization is a lot more complex than I imagined. Makes sense why companies take so long to return products sent for sterilization.

Scalability
Sterilization is a lot more complex than I imagined. Makes sense why companies take so long to return products sent for sterilization.

aftsnvvurj
how do you decide which method is the best for my product?
Aug 23, 2023
Consistent performance and uptime ensure efficient.

Scalability
Consistent performance and uptime ensure efficient.

juhatopi
EO is the best for sure, not sure why you don't promote it more
Aug 23, 2023
Like

Scalability
Reply

famat96602
What does Regulatory submissions and approvals actually involve?
Aug 18, 2023
Don't I already have all the approvals I need?

Reply
Like