Lets not forget STEAM is really HOT water…
Water is a universal solvent that has numerous applications. It is also the safest for use in all applications.
HOTTTT STEAM is lethal.
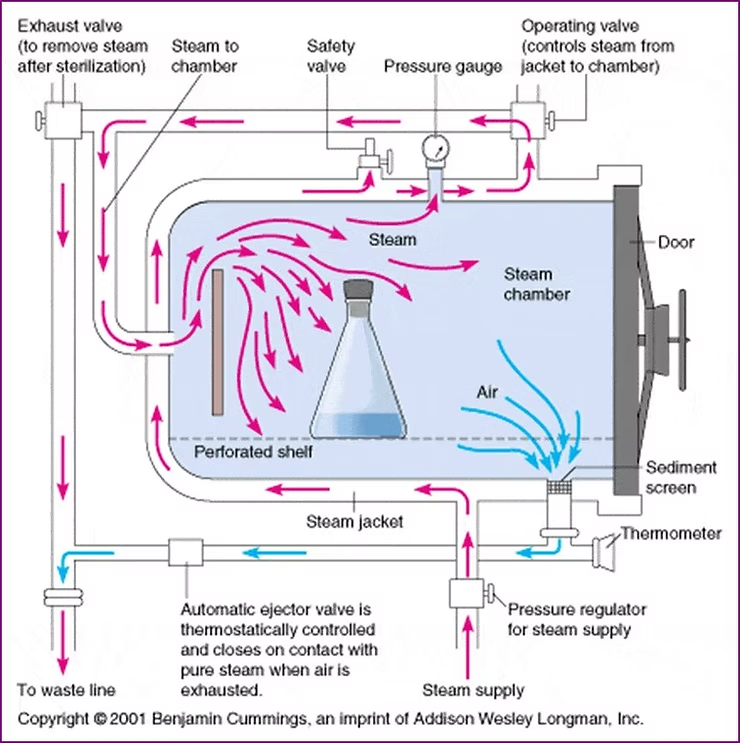
Historically, steam used in sterilization is pure water that is made really hot… 121°C hot…
Aug 23, 2023
3 min read
Source: wikipedia.org
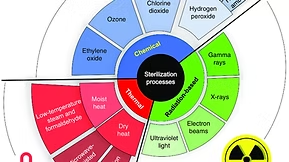
Steam allows release methods
Parametric release allows to replace sterility testing with acceptance criteria for process parameters per #ISO13485.
Are Biological indicators obsolete? Comment below.
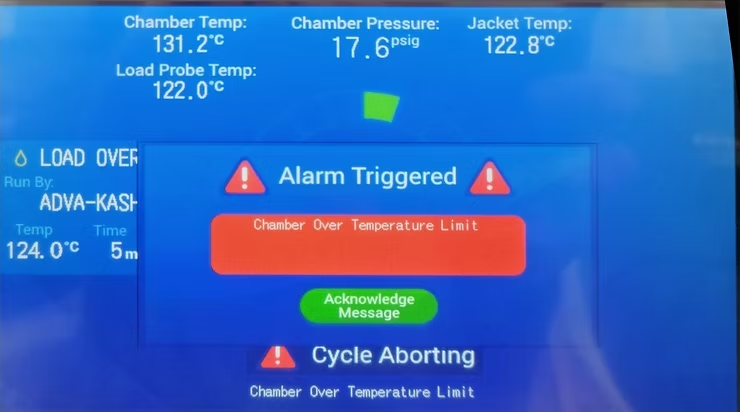
Automated protection for products and machine

Production process includes steps to avoid risks

Skilled staff to manage and check each process
How do we fit into all this?
Advasaf Sterilization provides contract sterilization, laboratory testing, and product and packaging testing services to manufacturers of medical devices, pharmaceuticals, consumer goods, and industrial products.
We support our Customers throughout the sterilization process with a comprehensive offering of testing and validation services, including microbiological, analytical, and product and packaging testing.
ISO 13485:2016 Certified Medical Device Quality Management System certification ensures that your product will benefit overall performance by consistently providing safe and qualitative medical devices and services.

Reliability
Optimal steam sterilization ensures reliable medical device processing with effective moist heat methods.

ISO Compliance
Speed and efficiency ensure tasks are completed quickly and resources are used optimally, enhancing productivity and satisfaction.

Scalability
Growth capability is a system's ability to scale and adapt, meeting increasing demands and evolving needs for long-term success.

Scalability
Meeting ISO13485 standards ensures our autoclaves are suited for sterilization in medical device industries.
Read customer opinions
Customers reviews and testimonials for a better understanding of our offers.